Test Instructions
The following are mannitol challenge test instructions developed for use with Aridol in Australia. For approved Aridol/Osmohale test instructions for your country please refer to the appropriate country specific homepage by selecting Production Information
Aridol Test instructions for Australia:
Aridol® Challenge
Challenge Outcomes
Positive Aridol® Challenge result
A positive Aridol® response may be achieved in 2 ways:
>= 15% fall in FEV1 from baseline (using the post 0mg FEV1 as comparator)
>= 10% incremental fall in FEV1 (between consecutive Aridol® doses)
Negative Aridol® Challenge Result
An Aridol® challenge is considered to be negative when a cumulative dose of 635 mg of Aridol® has been administered and the subject's FEV1 has not fallen by >=15% from baseline.
Equipment
Aridol® Kit (containing Aridol® capsules, an inhaler device and instruction leaflet)
Spirometer & mouthpiece
Nose clip
Timer (which can be set to 60 seconds)
Calculator
Bronchodilator (eg salbutamol) & volumatic spacer (if using a metered dose inhaler)
Oxygen and other relevant emergency equipment should be readily available as per standard Bronchial Provocation Testing protocols.
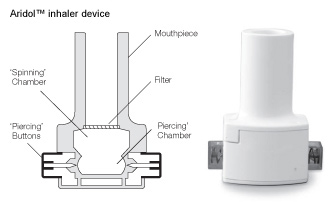
Inhaler Instructions
These instructions show you how to use the inhaler device included in the Aridol test kit
 |
1. Remove Cap: Using both hands, hold the inhaler upright and remove the cap |
 |
2. Open: Hold the base of the inhaler firmly with one hand and open the device by rotating the mouthpiece in the direction of the arrow as shown |
 |
3. Load: Using dry hands, remove a capsule from the Aridol™ pack and place into the inhaler as illustrated. It does not matter which way the capsule is placed in the chamber. |
 |
4. Close: Keeping the device in an upright position, twist the mouthpiece into the closed position until you hear it 'click'. |
 |
5. Pierce Capsule: Hold the inhaler upright and fully depress both piercing buttons on the sides of the device at the same time. |
 |
6. Prepare for Inhalation: Tilt the inhaler so that the mouthpiece faces slightly downward at a 45 degree angle as shown. This allows the capsule to drop forward into the spinning chamber. Keep the device tilted in this way and instruct the subject to breathe out completely (away from the inhaler). |
 |
7. Inhale: The subject should tilt their head back slightly, and keeping the inhaler at a 45 degree angle, raise the device to their mouth and ensure they close their lips tightly around the mouthpiece. Encourage the subject to take a controlled deep inhalation until their lungs are full. The subject should then hold their breath for five seconds. Note: During a successful inhalation you should hear a rattling sound as the capsule spins in the inhaler. |
 |
8. Exhale: Remove the inhaler from the subject's mouth, allow them to exhale and resume normal breathing. |
 |
9. Check: The Aridol® capsule must spin in the inhaler in order to empty. A second inhalation (using the same capsule) may be required immediately if the capsule has not been emptied sufficiently following inhalation. Check the capsule following each inhalation. |
 |
 |
 |
| 1. Remove Cap: Using both hands, hold the inhaler upright and remove the cap | 2. Open: Hold the base of the inhaler firmly with one hand and open the device by rotating the mouthpiece in the direction of the arrow as shown | 3. Load: Using dry hands, remove a capsule from the Aridol™ pack and place into the inhaler as illustrated. It does not matter which way the capsule is placed in the chamber. |
 |
 |
 |
| 4. Close: Keeping the device in an upright position, twist the mouthpiece into the closed position until you hear it 'click'. | 5. Pierce Capsule: Hold the inhaler upright and fully depress both piercing buttons on the sides of the device at the same time. | 6. Prepare for Inhalation: Tilt the inhaler so that the mouthpiece faces slightly downward at a 45 degree angle as shown. This allows the capsule to drop forward into the spinning chamber. Keep the device tilted in this way and instruct the subject to breathe out completely (away from the inhaler). |
 |
 |
 |
| 7. Inhale: The subject should tilt their head back slightly, and keeping the inhaler at a 45 degree angle, raise the device to their mouth and ensure they close their lips tightly around the mouthpiece. Encourage the subject to take a controlled deep inhalation until their lungs are full. The subject should then hold their breath for five seconds. Note: During a successful inhalation you should hear a rattling sound as the capsule spins in the inhaler. | 8. Exhale: Remove the inhaler from the subject's mouth, allow them to exhale and resume normal breathing. | 9. Check: The Aridol® capsule must spin in the inhaler in order to empty. A second inhalation (using the same capsule) may be required immediately if the capsule has not been emptied sufficiently following inhalation. Check the capsule following each inhalation. |
Important Points to Note
a. The inhaler device is designed as SINGLE USE ONLY (one device per challenge) and should not be cleaned during the challenge. Discard following each Aridol® challenge. Do not sterilise and re-use as this may compromise the integrity of subsequent test results.
b. When subject's are exhaling during the Aridol® challenge, ensure they do so AWAY from the inhaler to minimise humidity within the device.
c. When piercing the capsule, do so once only (by depressing both buttons simultaneously and fully) as re-piercing may cause the capsule to split/fragment.
d. Using latex gloves when administering the test and handling Aridol® capsules may increase static and inhibit capsule movement within the inhalation device.

e. If you suspect that static is an issue or notice that the sound of the capsule 'rattling' cannot be heard during inhalation of Aridol®, firmly tap the base of the inhaler with one hand whilst holding it with the other (mouthpiece facing downwards at a 45° angle). This should ensure that the capsule has been 'dislodged' from the piercing chamber into the spinning chamber.
f. Inhalation of Aridol® may cause cough and/or a dry throat. This is a routine consequence of bronchial challenge testing. You can offer the subject water to sip during/after the challenge.
g. This challenge test is time critical and requires an osmotic gradient to be established and maintained. Prolonged time intervals between doses may affect the validity of results and should be avoided.
Procedure Guidelines
STEP 1: Ensure subject has withheld medications as required (See table below).
Recommended Medication Withholding Times
Failure to withhold medications may affect the results of the Aridol® challenge. Recommended periods for withholding medications are generally based on their duration of action.
| Time to withhold | Medication |
|---|---|
| 6-8 hours | INHALED NON-STEROIDAL ANTI INFLAMMATORY AGENTS eg sodium cromoglycate, nedocromil sodium |
| 8 hours | SHORT-ACTING BETA2 AGONISTS eg salbutamol, terbutaline |
| 12 hours | INHALED CORTICOSTEROIDS eg beclomethasone dipropionate, budesonide, fluticasone propionate, ciclesonide and mometasone furoate |
| 12 hours | IPRATROPIUM BROMIDE |
| 24 hours | INHALED CORTICOSTEROIDS PLUS LONG-ACTING BETA2 AGONISTS eg fluticasone and salmeterol, budesonide and eformoterol |
| 24 hours | LONG -ACTING BETA2 AGONISTS eg salmeterol, eformoterol |
| 24 hours | THEOPHYLLINE |
| 72 hours | TIOTROPIUM BROMIDE |
| 72 hours | ANTIHISTAMINES eg cetirizine, fexofenadine and loratadine |
| 4 days | LEUKOTRIENE-RECEPTOR ANTAGONISTS eg montelukast sodium |
Foods: Ingestion of caffeine containing foods such as coffee, tea, cola and chocolate may decrease bronchial hyperresponsiveness. These substances should be withheld on the day of the test.
Other factors that may confound results: Smoking and vigorous exercise should not be undertaken on the day of the test.
STEP 2: Subject should be seated for the test. Explain the procedure; include what is required for an FVC manoeuvre and FEV1 measurement and the type of inspiratory flow required for the inhaler. Demonstrate as required.
STEP 3: Enter the subject's details in the spirometer as applicable (age, height, race, date of birth, gender etc).
STEP 4: Determine the pre-challenge FEV1. Ask the subject to perform 3 x repeatable FVC manoeuvres according to the ATS/ERS guidelines. The subject's FEV1 should be >=70% predicted. Caution should be used in subjects with an FEV1 of less than 70% predicted.
STEP 5: Calculate the baseline FEV1 (0 mg)
a. Remove the 0 mg Aridol® capsule from the blister, twist open the inhaler (as per the arrow on the device), place the capsule inside and close the device.
b. Pierce the capsule once only by depressing the coloured buttons on either side of the inhaler.
c. Ask the subject to put on the nose clip, and breathe through their mouth.
d. Tilt the inhaler at a 45° angle (mouthpiece down). Check the capsule has moved from the piercing chamber into the spinning chamber closest to the mouthpiece. You can often hear the capsule fall forward or see the capsule through the vents on each side of the device. Give the inhaler to the subject ensuring that they keep it at the same angle.
e. Ensure the subject is sitting up straight. Ask the subject to exhale (away from the inhaler), seal their lips around the mouthpiece and take a controlled deep inhalation until their lungs are full. During successful inhalation you should hear a 'rattling' sound as the capsule spins within the device.
f. At the end of the subject's inhalation, start a 60 second timer, and ask the subject to hold their breath for 5 seconds. When 5 seconds has passed, instruct the subject to exhale through their mouth (away from the inhaler), remove the noseclip and breathe normally.
g. When the timer beeps after 60 seconds, immediately instruct the subject to perform two repeatable FEV1 measurements. These measurements must be within 0.15L (150 mL) variability. If there is greater than 0.15L variability between readings instruct the subject to perform another FEV1. Record the highest FEV1 reading as the baseline FEV1. If the highest FEV1 is >=10% drop from the pre-challenge FEV1 do not continue with the test.
h. Calculate the target FEV1 A positive Aridol® challenge result is achieved when the subject's FEV1 falls >=15% from their baseline FEV1. To calculate the target FEV1, multiply the baseline FEV1 (the highest reading obtained at 0mg) obtained above by 0.85. Record this value.
STEP 6: 5mg capsule
a. Insert 5mg capsule into the inhaler and pierce as in Step 5.
b. Repeat as in steps c - f above.
c. Following inhalation remove the capsule from the inhaler and check to ensure it has been emptied sufficiently, if not, a 2nd inhalation will be required immediately.
d. Load the 10 mg capsule in readiness for the next dose.
e. At 60 seconds following inhalation, immediately measure the subject's FEV1 twice (repeatability criteria must be met). Use the highest of these two values to calculate the change in FEV1.
f. Compare the FEV1 value at this dose to the target FEV1. If the FEV1 value is equal to or below the target value, or there has been an incremental fall of >= 10% from the previous dose, the challenge is positive and complete. If not, immediately proceed to next dose step.
STEP 7: 10 mg, 20 mg, 40 mg capsules
Administer the 10mg, 20mg and 40mg doses following the directions given above (in step 6) for the 5mg dose.
STEP 8: 80mg dose (2 x 40 mg capsules)
a. Insert and pierce the first of the 40 mg capsules that comprise the 80mg dose.
b. The subject should inhale the dose in the same manner as previous doses, hold their breath for 5 seconds and exhale.
c. Remove the first 40mg capsule from device and check to ensure it has been emptied sufficiently if not, a 2nd inhalation will be required immediately. Do this following the administration of every capsule.
d. Following inhalation, load the second 40 mg capsule and offer to the subject immediately following exhalation.
e. Instruct the subject to inhale the 2nd capsule immediately to ensure that the osmotic effect or Aridol® is cumulative.
f. Activate timer at the end of the 2nd capsule inhalation.
g. Instruct the subject to hold their breath for 5 seconds before exhaling.
h. At 60 seconds following inhalation of the 2nd capsule, immediately measure the subject's FEV1 twice (repeatability criteria must be met). Use the higher of these two values to calculate the change in FEV1.
i. Compare the FEV1 value at this dose to the target FEV1. If the FEV1 value is equal to or below the target value, or there has been an incremental fall of >= 10% from the previous dose, the challenge is positive and complete. If not, immediately proceed to next dose step.
Step 9: 1st of 160 mg dose (4x 40 mg capsules)
a. Insert and pierce the 1st of the 40 mg capsules that comprise the 160 mg dose.
The subject should inhale the dose in the same manner as previous doses, hold their breath for 5 seconds and exhale.
b. Remove capsule from device and check to ensure it has been emptied sufficiently, if not, a 2nd inhalation will be required immediately. Do this following the administration of every capsule.
c. Following inhalation, load the 2nd 40 mg capsule and offer to the subject immediately following exhalation.
d. The subject should inhale contents of the 2nd capsule, hold their breath for 5 seconds and exhale.
e. Following inhalation, load the 3rd 40 mg capsule and offer to the subject immediately following exhalation.
f. The subject should inhale the contents of the 3rd capsule, hold their breath for 5 seconds and exhale.
g. Immediately following inhalation, load the 4th 40 mg capsule and offer to the subject immediately following exhalation.
h. Instruct the subject to inhale the 4th capsule immediately to ensure that the osmotic effect of Aridol® is cumulative.
i. Activate timer at the end of the 4th capsule inhalation.
j. Instruct the subject to hold their breath for 5 seconds, before exhaling.
k. At 60 seconds following inhalation of the 4th capsule, immediately measure the subject's FEV1 twice (repeatability criteria must be met). Use the higher of these two values to calculate the change in FEV1.
l. Compare the FEV1 value at this dose to the target FEV1. If the FEV1 value is equal to or below the target value, or there has been an incremental fall of >= 10% from the previous dose, the challenge is positive and complete. If not, immediately proceed to next dose step.
Step 10: 2nd x 160 mg dose (4 x 40 mg capsules)
Administer the 2nd 160 mg dose following the directions given above in step 9.
Step 11: 3rd x 160 mg dose (4 x 40 mg capsules)
Administer the 3rd 160 mg dose following the directions given above in step 9. At the completion of this dose, 635 mg has been administered. Providing a positive response has not been met, the challenge should be considered negative and complete.
Step 12: Following completion of the challenge with a positive result you should administer a bronchodilator and monitor the subject for 15 minutes to ensure their FEV1 has returned to within 5% of pre-challenge level. In the case of a negative result you may or may not wish to give a bronchodilator.
PLEASE REFER TO FULL PRODUCT INFORMATION BEFORE PERFORMING THIS CHALLENGE TEST.
For further information please consult your health care professional.
Aridol® is a registered trademark of Pharmaxis Ltd.
Pharmaxis Ltd, 20 Rodborough Road,
Frenchs Forest NSW 2086

